Browse Exhibits (11 total)
Airhenvbahihea Edionwe | Bacteriophage control of Salmonella enterica in artificially contaminated 1%, pasteurized milk

Salmonella spp. are the number one causative agent of foodborne illnesses in the United States each year. Salmonella spp. have been isolated from various food matrices including eggs, poultry, milk, and beef. Bacteriophage are viruses of bacteria. Bacteriophage, also known as phage, insert their genetic material into their hosts, hijacking their DNA synthesis machinery in order to produce more phage inside the host cell. Bacteriophage have been considered an alternative to antibiotics because of this lytic ability to kill their hosts. The goal of this study is to identify novel Salmonella bacteriophage from raw dairy milk and assess the ability to infect its host. For this experiment, three bacteria were used; Salmonella enterica subsp. Enteriditis, Salmonella enterica subsp. Pullorum, and S1 (an uncharacterized Salmonella species isolated from raw dairy milk. Salmonella enterica subsp. Enteriditis was used as the host for the novel bacteriophage. Upon phage isolation, enrichment is performed to increase phage titers. The phage is then added to various densities of bacteria to assess its ability to control growth under optimal conditions. Results from this experiment will lead an insight into better biological control methods.
Facult mentor: Dr. John L. Mckillip
Biology
Undergraduate
Delaney Long | Serotonin suppresses Drosophila serotonergic axon development in vitro
Serotonergic neurons modulate brain activity by releasing the neurotransmitter serotonin onto other neurons through long cable-like processes called axons. In order to successfully do this, axons from serotonergic neurons must precisely reach target neurons. If not achieved, malformed serotonergic axons can cause behavioral disorders impacting mood, sleep, and appetite. Assisting in this, serotonin regulates the development of axon structure in addition to its role as a neurotransmitter. Neurons unable to synthesize serotonin develop more branches and longer axons, suggesting it normally limits branching and elongation. Despite this observation, the molecular mechanism of this process, termed serotonin autoregulation, remains unknown. Here we developed a novel culture method allowing identification and analysis of developing primary Drosophila serotonergic neurons. To validate the utility of the culture system, we treated them with extracellular serotonin and found that high levels of serotonin inhibited axon growth. This is the first demonstration of serotonin autoregulation in a culture system, and combined with the powerful genetic tools available in Drosophila will provide exciting new insights into the molecular mechanism of serotonin autoregulation of serotonergic axon development.
Faculty Mentor: Douglas Roossien
Department of Biology
Graduate Student
Ellen Doss | Protein Quality Control in Candida albicans

Candida albicans is an opportunistic fungal pathogen that resides as normal flora in the human gastrointestinal tract and mouth. However, upon a patient’s immunosuppression due to a preexisting condition or an organ transplant, the yeast can colonize nearly every tissue and organ, causing life-threatening infections. Few treatment options exist for Candida infections; therefore, it is essential to understand the molecular mechanisms that contribute to its virulence. We are characterizing the roles of four genes involved in protein quality control, which is uncharacterized in C. albicans. Using CRISPR, we are generating homozygous deletions of HRD1, DOA10, UBC7, and STE24. These knockout strains are being characterized and compared to wild type yeast using growth assays to determine how well they grow in the presence of compounds predicted to increase the abundance of misfolded proteins. A virulence assay will then be performed utilizing a characterized insect infection model, Galleria mellonella wax moth larvae. Survival of larvae injected with each knockout strain will be compared to that of larvae injected with the highly virulent wild type strain. These experiments represent a novel investigation of protein quality control in C. albicans and have the potential to reveal new therapeutic targets for fungal infections.
Faculty Mentors: Dr. Eric (VJ) Rubenstein, Dr. Douglas Bernstein
Department of Biology
Graduate
Isabelle Wright | Olfactory Recognition in Brain Glycogen Knockout Mice

Glucose stored in the brain as the branched polysaccharide glycogen has been reported to play a role in associative learning. The effect of brain glycogen levels on sensory learning in mus musculus is rarely studied and, as a model species for human studies, has implications for learning in people, especially those experiencing low glucose availability to the brain. Wild-type mice and mice without brain glycogen were allowed to investigate the scent of a fruit juice for 5 minutes after a habituation period. Twenty-four hours later, the mice were allowed to investigate the scent of the same juice or a novel juice. The amount of time the mouse spent “exploring” the scent was measured on both days, and the times were compared between genotypes with the hypothesis that wild type mice would spend less time than knockout mice on a familiar scent 24 hours later. However, analysis of this data shows similar results between the two genotypes, implying that brain glycogen may not have a significant impact on sensory learning. When compiled with other behavioral studies with brain glycogen variables, this study improves understanding of the importance of mammalian brain glycogen levels for behavioral learning.
Faculty Mentor: Bartholomew A. Pederson
Biology
Undergraduate
Honors College
Jenna McKune | Elevated Plasma Creatinine Levels in Old Female Retinal Dystrophic Pigmented Royal College of Surgeon (RCS) Rats
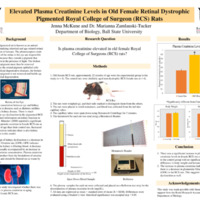
The Royal College of Surgeons (RCS) pigmented rat is known as being an animal model for studying inherited retinal degeneration seen in humans, and kidney problems are seen with eye problems. There is no information on kidney function in this model, so the purpose of this study was to investigate whether there are also kidney problems in this model. A decrease in glomerular filtration rate (GFR) is seen when there is kidney dysfunction and is usually accompanied by an increase in the plasma creatinine concentration; there was a significant increase in the plasma creatinine concentration in the old female RCS rats compared to their control counterparts, indicating they have kidney issues in combination with their eyes.
Faculty Mentor: Dr. Marianna Zamlauski-Tucker
Department of Biology
Undergraduate
Kamryn Kennedy | Ste24 Substrate Specificity in Translocon Quality Control
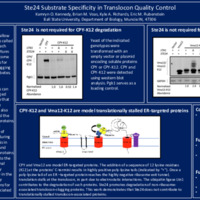
Translocons are molecular channels that allow proteins to cross membranes in a process called translocation. This enables proteins to reach their appropriate cellular locations to perform their specific functions. If translocons become clogged, they cannot be used as passages for other proteins. Unresolved translocon clogging may result in diseases, such as type 2 diabetes. There are two modes of translocation, posttranslational translocation (PTT) and cotranslational translocation (CTT). Errors can occur in both processes, resulting in clogged translocons. Ste24 is a conserved enzyme that degrades clogging proteins that enter the translocon via PTT. It is unknown if Ste24 also degrades proteins that use CTT. We tested the hypothesis that Ste24 degrades clogged proteins that undergo CTT, using a Saccharomyces cerevisiae model system. The abundance of proteins engineered to clog translocons during CTT was analyzed via western blot analysis in yeast containing or lacking STE24 . We found Ste24 does not promote degradation of proteins that undergo CTT. These data contribute to the knowledge of how cells maintain functional translocons, suggesting translocon quality control enzymes exhibit strong specificity in the types of translocon clogging proteins they degrade. Our results may have implications for understanding and treating diseases associated with translocon dysfunction.
Faculty Mentor: Eric Rubenstein
Department of Biology
Undergraduate
Kyle Richards | Translocation Through the Endoplasmic Reticulum Translocon is Impaired by Translocon Modification

The endoplasmic reticulum (ER) is the entry point for most proteins residing and functioning in the eukaryotic endomembrane system. The primary mechanism by which proteins enter the ER is via the translocon complex. Dysfunction in this complex can block access into the ER, which is detrimental to cellular health. The translocon is highly conserved and has been intensely studied in Saccharomyces cerevisiae. Analysis of translocon function in protein trafficking, localization, and interactions in yeast has been facilitated by the use of epitope tags. We have found that a tag on the translocon pore subunit previously suggested not to impair translocon function subtly affects translocation of proteins into the ER in yeast cells. Intriguingly, this tag also suppresses a phenotype associated with defective protein quality control pathways, consistent with a functional link between translocation and quality control. Ongoing work includes characterizing the effects of placing different epitope tags on different translocon subunits, with the goal of identifying tags that affect translocon function the least.
Faculty Mentor: Eric VJ Rubenstein
Department of Biology
Graduate
Maia Campbell, Ally Lankford | Temperature Controls on Microcystin Degradation
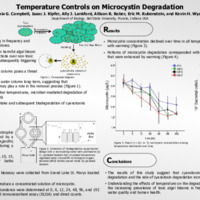
Toxic cyanobacteria blooms are increasing in frequency and severity with rising surface-water temperatures. Warming generates a positive feedback on harmful algal bloom development by promoting toxic cyanobacteria over non-toxic strains, stimulating toxin synthesis, and subsequently triggering toxin release. The release of cyanotoxins into the water column poses a serious threat to water quality and human health. Yet, cyanotoxins rarely accumulate in the water column long-term, suggesting that biodegradation by heterotrophic bacteria may play a role in the removal process. Owing to increased metabolic rates at higher temperatures, microbial-mediated degradation of cyanotoxins may be influenced by warming. However, the effect of warming on the uptake and subsequent biodegradation of cyanotoxins has not yet been evaluated. In this study, we evaluated the ability for heterotrophic bacteria to degrade microcystin produced by a common cyanobacterium, Planktothrix agardhii, across a natural temperature gradient (from 5 to 20° C) during a laboratory incubation experiment. We measured microcystin concentration and bacterial abundance at 0, 6, 12, 24, 48, 96, and 192 hours. We found that microcystin concentration declined over time in all temperature treatments and degradation increased with warming. Patterns of microcystin degradation corresponded with increases in bacterial cell density over time that were enhanced by warming. Bacterial cell density increased most rapidly in the 20°C treatment resulting in the greater microcystin degradation than all other treatments. Degradation of microcystin was lowest in the 5°C treatment where the increase in bacterial cell density was the slowest. Bacterial density and microcystin degradation were similar between 10°C and 15°C treatments throughout the experiment and were consistently between 20°C and 5°C treatments. These results suggest that cyanotoxins can be reduced by microbial-mediated degradation and the rate of cyanotoxin degradation increases with water temperature.
Faculty Mentors: Dr. Allison R. Rober, Dr. Kevin H. Wyatt
Department of Biology
Undergraduate
Samantha Turk | Determining the Role of a Transcription Factor in Protein Degradation

Proteins are essential to life. They perform a variety of functions within the cell, including cell regulation and DNA synthesis. Just as important as protein synthesis is the antiparallel process of protein degradation. A protein must be degraded when it is no longer necessary, is damaged, or behaves aberrantly to prevent organismal harm. Proteins can behave aberrantly by persistently engaging with a protein channel called the translocon, which allows proteins to move across the membrane of the endoplasmic reticulum. In humans, a protein known to clog the translocons is a component of low-density lipoproteins (or “bad cholesterol”). A ubiquitin ligase in yeast known as Hrd1 polyubiquitylates the aberrant protein, tagging it for degradation via the proteasome. The proteasome detects polyubiquitination and degrades tagged proteins, recycling them into shorter fragments. Ubiquitin ligases rarely function alone, and yeast lacking Hrd1 still exhibit residual degradation of translocon-clogging proteins, suggesting the existence of alternative degradation pathways. We performed a genome-wide screen to identify genes that may play a role in protein degradation of translocon-clogging proteins, identifying a potential 150 candidates. Further small-scale reporter assays were performed, confirming the role for 42 genes in protein degradation. Additional biochemical validation using cycloheximide chase showed the requirement of 3 genes, one of which is part of a heterodimeric transcription factor complex involved in lipid synthesis. With the process of protein degradation being conserved in both yeast and humans, validated genes may represent therapeutic targets for patients with elevated levels of cholesterol.
Faculty Mentor: Dr. Eric "VJ" Rubenstein
Department of Biology
Graduate
Sydney Oliver | Understanding how MitoNEET Contributes to Oxidative Stress
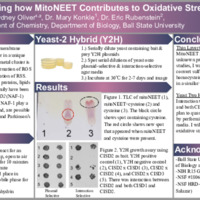
MitoNEET is a outer mitochondrial membrane protein that contains a [2Fe-2S] cluster. Since its discovery, three genes of the protein family have been identified as CISD1 (mitoNEET), CISD2 (NAF-1), and CISD3 (Miner 2). If the metal cluster is lost from NEET proteins, it can cause oxidative stress because free iron causes the formation of reactive oxygen species (ROS) which can then go on to cause the formation of reactive sulfur species (RSS). These reactive species cause damage to proteins, lipids or DNA. MitoNEET and NAF-1 play unclear role(s) in resistance to oxidative stress and are possible drug targets for cancer cell, diabetes, and Parkinson’s Disease. In my project I have started to explore how 1.) mitoNEET contributes to sulfur-metabolism in human biochemistry and 2.) how NEET proteins interact with each other. This study is impactful because oxidative stress in cells is strongly influenced by the balance between ROS and RSS. I am completing this project to understand how the chemistry of mitoNEET impacts RSS and then extending this knowledge into yeast cells to examine the results in a cellular environment under oxidative stress. The primary research methodology of thin-layer chromatography showed that a new chemical is being made when the amino acid cysteine is combined with mitoNEET. This result indicates an enzymatic function of mitoNEET that would integrate into maintaining cellular redox balance. Additionally, yeast-2 hybrid experiments have shown an interaction between NAF-1 with itself and NAF-1 with mitoNEET. Future studies will extend this work by examining how NAF-1 interactions in yeast cells are impacted by oxidative stress.
Faculty Mentor: Mary Konkle
Biology
Graduate
