Browse Exhibits (1 total)
Kwabena Duah, Lauren Andrews | Synthesis of Open-Chain Analogue of Ipomoeassin F
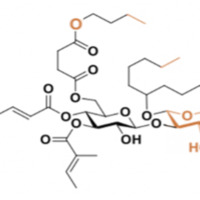
Resin glycosides are the active ingredient in most morning glory-based medicines. They possess numerous biological applications such as laxative, purgative, antibacterial, and antifungal properties. Ipomoeassin F is a novel glycoresin that was isolated from Ipomoea squamosa, and it has a unique structure and anticancer activities with IC50 values in the low nanomolar range for most cancer cell lines. Ipomoeassin F’s mechanism is underexplored due to the structural complexity, large time requirements, and high costs. In order to deepen our understanding of how ipomoeassin F works, decrease cost, labor, and time, we are synthesizing a novel simplified open-chain analogue 1 of ipomoeassin F from readily available D-glucose and L-arabinose. This structure was designed based on previous structure-activity relationship (SAR) studies. Analogue 1 is synthesized by utilizing glycosylation followed by esterification and deprotection reactions to couple the glucosyl donor, (synthesized from D- glucose over 7 linear steps) and the arabinose acceptor (synthesized from L-arabinose over 7 linear steps). Both the glucosyl donor and the arabinose acceptor have been fully synthesized in excellent yields, and the synthesis of analogue 1 is still ongoing. Once synthesis of the target analogue 1 is completed, it is intended to be assayed for biological potentials which could then fuel the design and synthesis of future analogues. This can then be advanced into the field of drug discovery.
Faculty Mentor: Dr. Wei Q. Shi
Department of Chemistry
Undergraduate/Graduate
Honors College
