Browse Exhibits (4 total)
Emma Cieslik | Hot Button Issue: Material Composition Analysis of Archaeological Ceramic Buttons

This research analyzed 10 buttons recovered from an archaeological site for composition. The buttons were excavated from an unmarked 19th century African American cemetery in Lowndes County, Mississippi. Each button was sampled, and infrared spectra were obtained for each sample using Fourier Transform infrared spectroscopy. The unprocessed spectra were deconvolved, and a fourth derivative of spectra were obtained to check the validity of the peaks. Replicate spectra of each button were also obtained.
Preliminary project results indicate that the majority of button samples may be composed of a mixture of different types of feldspars, a group of technological minerals, clay, and sillimanite. Reference spectra for each feldspar type is very similar and makes distinct feldspar identification difficult. The spectral data indicate that these buttons are composed of materials consistent with the Prosser button method production. Prosser buttons were commonly used for clothing from 1840 onward (Sprague 2002). According to the patent, Prosser buttons were composed of flint and clays. Unlike the higher-quality Prosser buttons that were often made from ground quartz, these buttons were likely made of ceramic wasters, including a combination of the aforementioned feldspars.
These results contribute to the field of feldspar identification, material composition identification of archaeological artifacts, and the production profile of buttons dating to the Civil War period. Understanding button production provides interesting insight into industrial methods and clothing history.
Faculty Mentors: Dr. Patricia Lang (Department of Chemistry) and Dr. Homes Hogue (Department of Anthropology)
Department of Chemistry and Department of Anthropology
Undergraduate
Honors College
Jacob Wade | Overcoming Synthetic Challenges with Single-Atom Linked Dithiolate Bridged [Fe-Fe]-hydrogenase Mimic Compounds

Due to their high catalytic turnover frequency, [Fe-Fe]-hydrogenase mimic compounds have been suggested as potential replacements for platinum in the catalytic reduction of water into hydrogen for fuel cells which has fueled significant research into these compounds. The natural enzyme is believed to be a three-atom azadithiolate (adt) linked complex. There is an abundance of literature for the two-atom or larger linked mimic compounds, however only a relatively small amount exists which covers the single-atom linked compounds. Through thorough study of the literature, we have been able to successfully synthesize two single atom linked compounds: methanedithiolate (mdt) diironhexacarbonyl and diphenylmethanedithiolate (dpmdt) diironhexacarbonyl, with hopes to apply these routes to synthesize novel compounds. Though we have successfully synthesized these compounds, we have had difficulty with purifying them, always obtaining impure material from the silica gel column. We have successfully replicated the synthesis of these compounds in adequate yields with multiple side-products but have received inconsistent results during purification. One major challenge with these compounds is that these compounds are extremely non-polar, quickly eluting with 100% pentane/hexanes. These compounds are highly soluble in these solvents which complicates attempts at recrystallization. The presentation herein highlights the challenges which we have faced, and the steps taken to successfully synthesize and purify these compounds and plans to apply the learned techniques to synthesizing novel compounds including: di-2-pyridylmethanedithiolate (dpymdt) diironhexacarbonyl and the nitrogen based aminodithiolate (ndt) diironhexacarbonyl.
Faculty Mentor: Dr. Jesse Tye
Chemistry
Graduate
Kwabena Duah, Lauren Andrews | Synthesis of Open-Chain Analogue of Ipomoeassin F
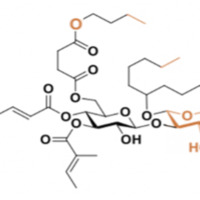
Resin glycosides are the active ingredient in most morning glory-based medicines. They possess numerous biological applications such as laxative, purgative, antibacterial, and antifungal properties. Ipomoeassin F is a novel glycoresin that was isolated from Ipomoea squamosa, and it has a unique structure and anticancer activities with IC50 values in the low nanomolar range for most cancer cell lines. Ipomoeassin F’s mechanism is underexplored due to the structural complexity, large time requirements, and high costs. In order to deepen our understanding of how ipomoeassin F works, decrease cost, labor, and time, we are synthesizing a novel simplified open-chain analogue 1 of ipomoeassin F from readily available D-glucose and L-arabinose. This structure was designed based on previous structure-activity relationship (SAR) studies. Analogue 1 is synthesized by utilizing glycosylation followed by esterification and deprotection reactions to couple the glucosyl donor, (synthesized from D- glucose over 7 linear steps) and the arabinose acceptor (synthesized from L-arabinose over 7 linear steps). Both the glucosyl donor and the arabinose acceptor have been fully synthesized in excellent yields, and the synthesis of analogue 1 is still ongoing. Once synthesis of the target analogue 1 is completed, it is intended to be assayed for biological potentials which could then fuel the design and synthesis of future analogues. This can then be advanced into the field of drug discovery.
Faculty Mentor: Dr. Wei Q. Shi
Department of Chemistry
Undergraduate/Graduate
Honors College
Phillip Betts | Development of High-Throughput Assays for the Detection of Rieske Dioxygenase Activity

Rieske dioxygenases are a class of enzymes found in soil bacteria, that are known to catalyze aromatic compounds via dihydroxylation to form enantiopure cis-diol metabolites. The regio-/stereoselective nature of enzymes make it a promising tool for synthetic chemistry by discovering new methods to form complex compounds.
Enzymes can be an environmentally friendly alternative to traditional catalysts by eliminating the need for petroleum based solvents, as enzymes can perform in aqueous solutions, and toxic heavy metals. However, enzymes are limited by their substrate scope and strict selectivity. In order to overcome this, protein engineering has been employed to expand the reactivity of enzymes.
To detect the activity of engineered dioxygenases, a novel assay system for the detection of aromatic substrate metabolites was developed. Here, the cis-diol metabolite was oxidize to form dialdehydes. These aldehydes can then conjugate with a fluorescent probe to give a strong fluorescent signal. However, this metaperiodate fluorescein cis-diol assay (MPFCD) does not give a strong fluorescent signal with aliphatic substrates. Thus, optimization of an assay for aliphatic dihydroxylation for metabolite detection via absorbance was investigated. These throughput assays can allow for distinct determination of engineered Rieske dioxygenase variants that show improved reactivity towards substrates not native to the enzyme.
Faculty Mentor: Dr. Jordan Froese
Department of Chemistry
Undergraduate
