Progress of Current Work
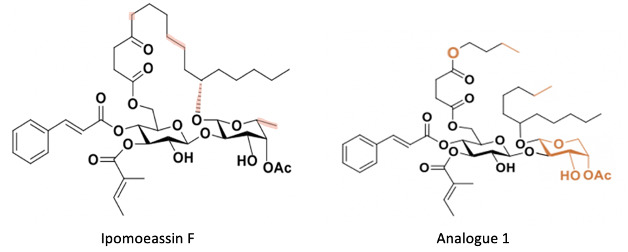
We are currently working on the synthesis of a ring-opened analogue of ipomoeassin F (figure 2, 5). Instead of using D-fucose, L-arabinose is utilized and is substantially cheaper (D-fucose is $115.87/g while L-arabinose is $6.55/g). By bypassing the ring-closing step higher yields can be achieved. Removal of the stereochemistry at C11 makes the synthesis less troublesome. Lastly, based on the previous SAR studies biological activity was retained when oxygen was added to the highlighted carbon. These are the four parts of ipomoeassin F that are changed in analogue 1.
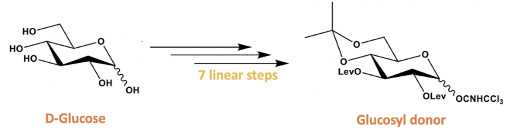
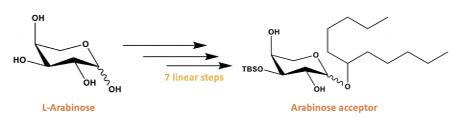
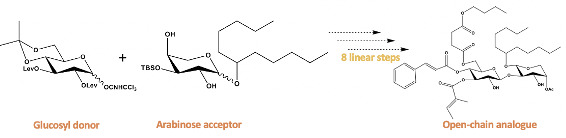
There are three components to our overall synthesis: synthesis of the glucosyl donor (figure 3), synthesis of the arabinose acceptor (figure 4), and completion of the analogue by utilizing glycosylation followed by esterification and deprotection reactions to couple the glucosyl donor and the arabinose acceptor (figure 5). Both the glucosyl donor and arabinose acceptor have been synthesized in excellent yields in our laboratory. However, the completion of the total synthesis is still ongoing.
Along with the goal of decreasing cost, we have decreased the labor required and time needed in the synthesis of the glucosyl donor, a very important component of ipomoeassin F. This is because previous syntheses of the glycosyl donor had four steps where column chromatography was required to clean the synthesized products11. This on its own involves the use of many organic solvents generating gallons of organic waste, takes several hours, and requires the sole attention of the researcher. Interestingly, in seven steps we have decreased the columns needed to clean our glucosyl donor molecule to only one column while increasing yield. This has dramatically cut down time required, reduced the cost of organic solvents, and of organic wastes.