Previous Structure-Activity Relationship Studies
Structure determines the function of all molecules. There have been numerous structure-activity relationship (SAR) studies of ipomoeassin F performed over the years. These are done in order to find a more efficient synthesis of ipomoeassin F and to help illuminate the mechanism of how it interacts with the cell. Once we understand this mechanism, we can determine what exactly needs to be edited in order to utilize ipomoeassin F to generate potent drugs that can help solve, or even cure, cancer and viral diseases. Each study is illustrated below.
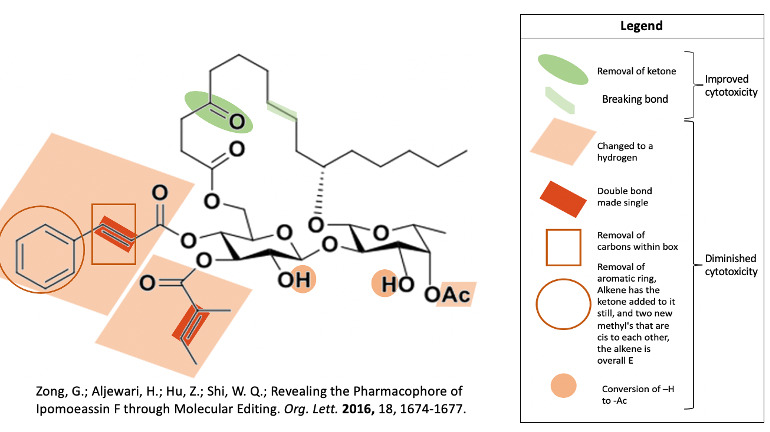
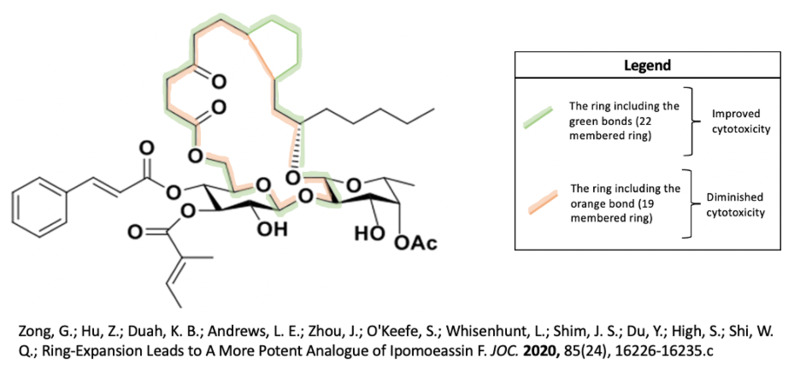
In a 2016 study by Zong and associates, they determined that removal of the ketone in the fatty acid tether or breakage of the C8-C9 bond led to retained and even improved cytotoxicity of ipomoeassin F6. Removal of that ketone even made the molecule better at differentiating between cancer cells and healthy cells6. They also discovered many components of ipomoeassin F’s structure that are crucial to its function, in order of importance: cinnamate, tiglate, phenyl, alkene in cinnamate, alkene in tiglate, acetate6.
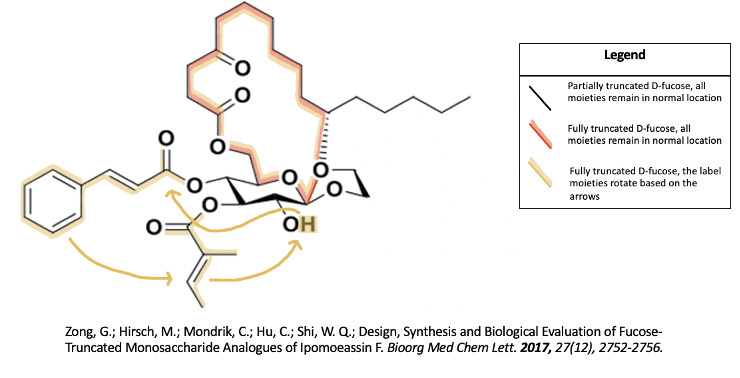
The 2017 study by Zong and associates investigated the significance of the fucose group by both partially truncating it and fully truncating it7. They discovered that the fucose group is essential to ipomoeassin F’s cytotoxicity7. They also said future studies in using a different sugar in place of fucose could help to decrease costs of production as D-fucose is an expensive reagent7.
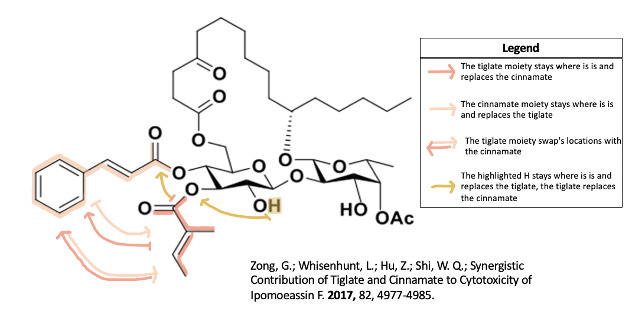
In Zong and associates' second 2017 paper they investigated if the tiglate and cinnamate are dependent on each other or if something else could be in place of one of them8. They made analogues with two tiglates or two cinnamates8 and they determined that the cinnamate and tiglate moieties work together and need to be on their appropriate carbon8. They also synthesized ipomoeassin F in a 4.0% yield which is the best to date8.
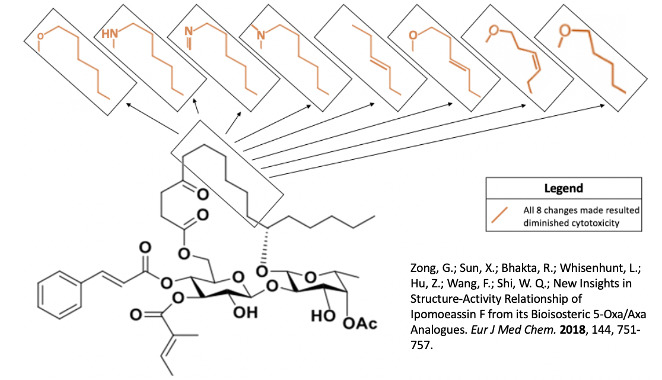
In a 2018 study by Zong and associates, they investigated the addition of an ester or nitrogen group in the fatty acid tether while also toggling with an alkene across the C8-C9 bond9. These changes did not necessarily increase the activity of ipomoeassin F, but they also did not greatly inhibit it either, suggesting this is a modifiable part of the structure9.